Welcome to part 6 of the ODX "Deep Dive Into Iron Metabolism" Series. In the 6th post in our series, we cover addressing disorders associated with altered iron status.
The primary approach to managing altered iron status involves increasing iron intake and availability for IDA while decreasing the body’s iron load in hemochromatosis and iron overload.
Inflammation must be considered when evaluating iron status as inflammatory cytokines shift iron from circulation and hemoglobin synthesis to storage as ferritin (Raymond 2021). It is essential to rule out the effects of inflammation on iron biomarkers before intervention, as it can cause a transient increase in serum ferritin accompanied by a decrease in transferrin, serum iron, and hemoglobin. Additional factors such as smoking, dehydration, and higher altitudes can increase hemoglobin and confound iron status evaluation as well (Pfeiffer 2017).
Dietary iron is consumed as heme iron from animal sources and non-heme iron from animal and plant-based sources. Heme iron is more easily absorbed, while non-heme iron is less bioavailable. Although non-heme iron is more susceptible to inhibitors in the diet, absorption can be improved by consuming ascorbate or animal tissue, including meat, fish, and poultry. However, some specific proteins can inhibit absorption (Abbaspour 2014).
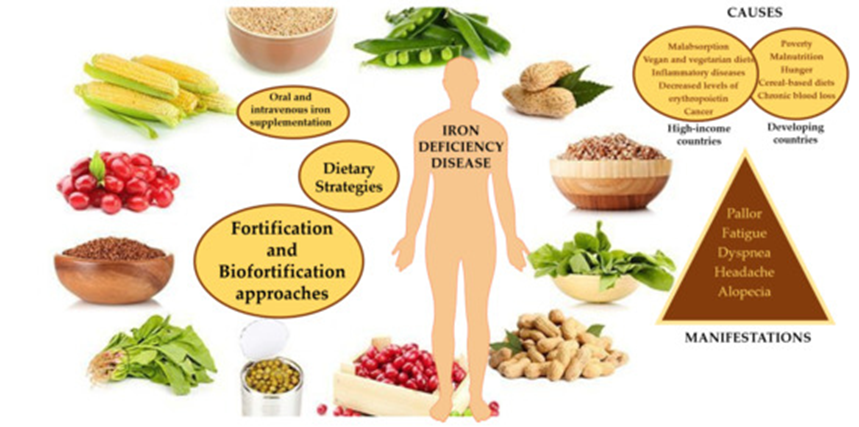
If dietary iron intake is inadequate, oral or intravenous iron supplementation may be indicated unless iron overload is present. Oral iron is readily used with uncomplicated iron deficiency and often includes ferrous sulfate, ferrous fumarate, and ferrous gluconate (Hanif 2022).
However, ferrous sulfate is likely to cause nausea or constipation. Instead, iron chelated with Krebs cycle intermediates or amino acids, such as ferrous glycinate or bis-glycinate, may be preferred due to fewer gastrointestinal side effects. Amino acid chelates are often preferred as they are less affected by compounds that inhibit iron absorption, e.g., calcium, phosphate, phytate, and oxalate. Iron supplementation should be accompanied by a balanced intake of micronutrients, including antioxidant nutrients such as vitamins A, C, and E, which can protect against the increased oxidative stress accompanying iron supplementation. Iron bioavailability can be increased from plant-based food by fermenting, sprouting, soaking grains, nuts, seeds, and legumes, and consuming heme iron at the same meal. Pretreatment with phytase enzymes can also increase iron bioavailability. Vitamin C increases the bioavailability of non-heme iron and should be consumed at all meals unless iron overload is present. Cooking in iron pans can also increase dietary iron absorption (Raymond 2021).
The status of other nutrients can affect iron metabolism as well. Zinc competes with iron for absorption and should be consumed separately, while riboflavin and vitamin A deficiencies can impair iron utilization and should be corrected to optimize therapy (Abbaspour 2014).
| Dietary Iron Absorption | ||
| Inhibited by | Competes with | Facilitated by |
| Alkaline GI pH Coffee, tea Divalent cations, including calcium, zinc, and manganese. Oxalates Phosvitin Phytates Polyphenols Tannins Specific proteins, including casein, whey, egg, albumin, and soybean |
Cobalt Lead Manganese Zinc |
Acids Acidic GI pH Ascorbate Citrate Fish Meat Mucin Poultry Sugars, e.g., fructose, sorbitol |
| Sources: (Abbaspour 2014, Gropper 2021) | ||
Liberal, Ângela et al. “Fighting Iron-Deficiency Anemia: Innovations in Food Fortificants and Biofortification Strategies.” Foods (Basel, Switzerland) vol. 9,12 1871. 15 Dec. 2020, doi:10.3390/foods9121871 This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
| Specific sources of iron and iron inhibitors | ||
| Heme iron | Non-heme iron | Inhibitors of non-heme iron |
| Oysters Liver Lean red meat Poultry, dark meat portion Tuna Salmon |
Iron-fortified cereals Dried beans/legumes Whole grains Nuts, especially almonds, Brazil nuts Dried fruits, especially prunes, raisins, apricots Vegetables, greens, especially broccoli, spinach, kale, collards, asparagus, and dandelion greens |
Oxalic acid in raw spinach, chocolate Phytic acid in wheat bran and legumes Tannins in black or pekoe tea Polyphenols in coffee Calcium in foods, supplements |
| Source: Raymond 2021 | ||
Reducing the iron load in the body is indicated for iron overload and may be achieved with phlebotomy if tolerated. Chelation therapy may be preferred if hemoglobin levels are too low to tolerate phlebotomy. Donating blood regularly may also reduce ferritin levels to acceptable levels (McDowell 2022).
Advanced stages of hemochromatosis may present with serum ferritin levels exceeding 1,000 mg/L. Treatment often includes weekly phlebotomy, removing 400-500 mL of blood, which can contain 200-250 mg of iron. Chelation of iron in the blood, followed by excretion in the urine, is also utilized. The goal is to decrease serum ferritin to 50-150 mg/L with a transferrin saturation below 45% (Gropper 2021).
NEXT UP: Iron Metabolism Part 7 - ODX Optimal Takeaways and References