Welcome to part 2 of the ODX "Deep Dive Into Iron Metabolism" Series. In the 2nd post in our series, we cover iron physiology.
Iron is an essential trace mineral for producing hemoglobin, myoglobin, and DNA. It also participates in mitochondrial electron transport, cell regulation, proliferation (Hanif 2022), and myelin production and maintenance. The iron in hemoglobin gives blood its red color and accounts for much of the iron in the body. However, hemoglobin-bound iron is unavailable for other functions, including mitochondrial energy generation and enzyme cofactor responsibilities. Serum iron measurement can assist in evaluating how much iron is available to tissues, including the brain, where it is required for energy-generating proteins (Kotze 2009).
The primary mechanism for balancing iron homeostasis is the regulation of intestinal absorption, as the body does not have a designated means to excrete iron. The peptide hormone hepcidin is the master regulator of iron absorption. Its presence blocks iron absorption, while its absence facilitates it. The failure to block iron absorption when needed leads to iron overload and vital organ damage, characteristic of hemochromatosis. On the other hand, overexpression of hepcidin can lead to insufficient iron absorption and anemia. Plasma iron, hypoxia, anemia, and cytokines all influence hepcidin levels. Chronic inflammatory states, including obesity, can increase hepcidin expression and contribute to iron deficiency. Gastric acid is essential for iron absorption and maintaining its divalent ferrous state. Reduction of gastric acid by proton pump inhibitors can substantially reduce iron absorption (Abbaspour 2014).
Hepcidin works closely with the protein ferroportin, which directly regulates iron absorption from food, the release of iron from the liver, and the recycling of iron from macrophages. Hepcidin causes the degradation of ferroportin, causing a decrease in iron absorption from food, including when iron stores are adequate. Hepcidin production is blocked when iron stores are low, allowing ferroportin to facilitate duodenal iron absorption. However, a prolonged absence of hepcidin can cause excess iron to be absorbed, leading to iron overload. This abnormality is seen with hereditary hemochromatosis and iron-loading anemias such as beta-thalassemia. Conversely, pro-inflammatory cytokines stimulate hepcidin production, resulting in decreased iron absorption and release from storage, leading to anemia of chronic disease, also known as inflammatory anemia. Abnormally elevated hepcidin is also seen with iron-refractory iron deficiency anemia (IRIDA, a rare condition (Gattermann 2021).
Free iron can cause excessive oxidative stress and must be bound to prevent free radical damage to tissues and organs. It can be bound to proteins, such as the heme group proteins in cytochrome and catalase enzymes, to ferritin in storage, or transferrin in circulation (Kotze 2009).
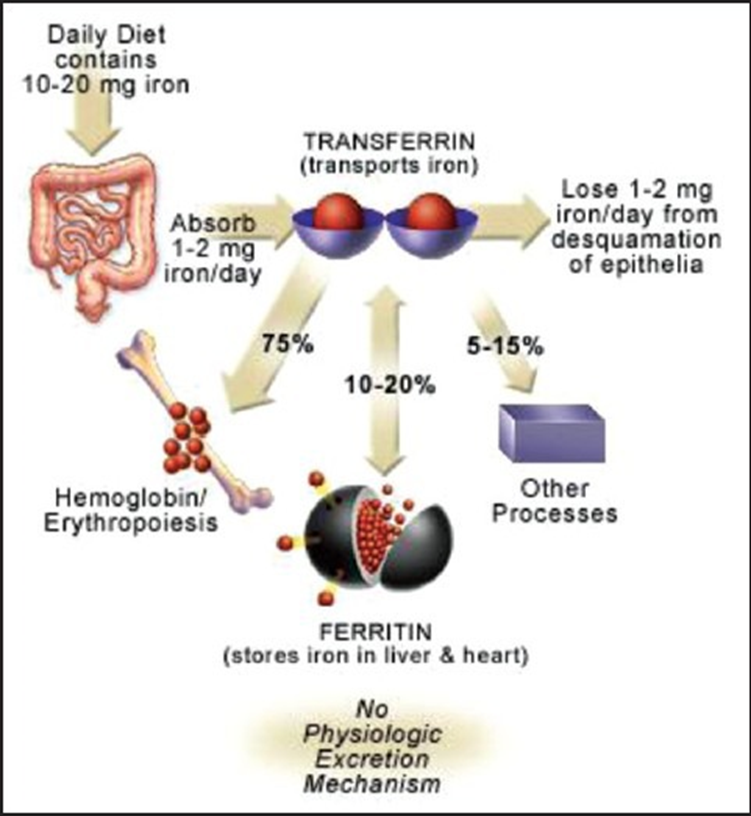
Iron is bound and transported in the body via transferrin and stored in ferritin molecules. Once iron is absorbed, there is no physiologic mechanism for excretion of excess iron from the body other than blood loss, that is, pregnancy, menstruation, or other bleeding.
Source: Abbaspour, Nazanin et al. “Review on iron and its importance for human health.” Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences vol. 19,2 (2014): 164-74. This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported.
NEXT UP: Iron Metabolism Part 3 - Disorders associated with altered iron status: Iron Deficiency Anemia