Thallium is a toxic metal that finds its way into the human body primarily through contamination from industrial sources such as mineral smelters and coal-burning facilities. It is more toxic than mercury, lead, or cadmium and affects humans, animals, plants, and microorganisms. It is passed on to humans through contaminated air, water, and food.
Although it is a toxin, thallium mimics potassium and can disrupt metabolic processes associated with potassium in the body. It also impairs several metabolic enzymes.
Thallium bioaccumulates (builds up) in the bones, kidneys, and nervous system and can cause:
Increased levels of thallium are found in vegetables, fruit, and farm animals, especially fungi, and vegetables in the Brassica family, including cabbage, kale, and rapeseed. The amount of thallium in cabbage increases as the pH of the soil decreases. Thallium accumulation in fish is especially high in those found near lakes subject to wastewater discharge from uranium milling.
In the United States, daily dietary intake of thallium is estimated to be 2 ppb, with an average content in the human body of 0.1 mg. The levels in blood can be as high as 3 ug/L, although normal total blood thallium is below 2 ug/L. However, calculated reference values range from 0.15 – 0.63 ug/L in blood and 0.02 – 0.34 ug/L in serum. Thallium levels can be three times higher in fingernails than in hair.
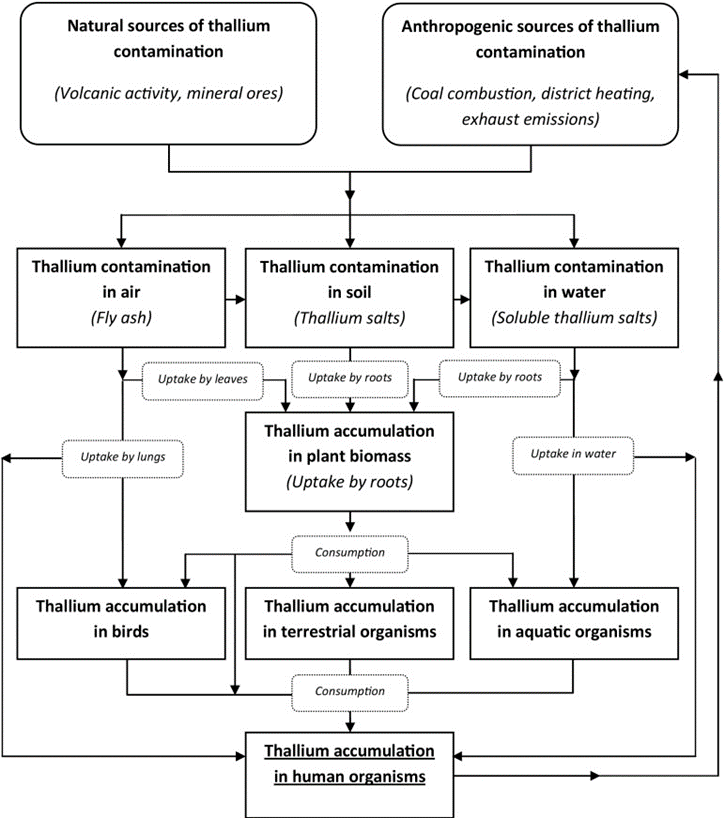
Karbowska, Bozena. “Presence of thallium in the environment: sources of contaminations, distribution and monitoring methods.” Environmental monitoring and assessment vol. 188,11 (2016): 640. doi:10.1007/s10661-016-5647-y This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/27783348/
The Quest Diagnostics reference range for whole blood thallium is below 5.1 ug/L
The ARUP reference range for whole blood thallium is below 2 ug/L
Thallium sulfate is used for rat and ant poison, and radioactive thallium-201 is used for nuclear stress tests for evaluating coronary artery disease. Cellular contamination with thallium and displacement of potassium with thallium can result in (Kemnic 2023):
Kemnic, Tyler R. and Meghan Coleman. “Thallium Toxicity.” StatPearls, StatPearls Publishing, 10 July 2023. https://www.ncbi.nlm.nih.gov/books/NBK513240/ This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ),