The detoxification or “biotransformation” of toxic compounds into something less harmful and more excretable requires several nutrients and plant-based compounds called phytochemicals or phytonutrients. Compounds that require detoxification include pharmaceuticals, hormones, bilirubin, heavy metals, and environmental pollutants such as pesticides and phthalates.
This metabolic detoxification occurs in steps and phases that depend heavily on amino acids, antioxidants, vitamins, minerals, and phytonutrients. Specific steps include phase 1 cytochrome P450 enzymes, phase 1 hydroxylation and reduction enzymes, phase 2 conjugation enzymes, antioxidant support, and metallothionein upregulation. In general, phase 1 can generate dangerous reactive compounds, phase 2 conjugates those compounds to make them less toxic, and antioxidants help protect tissues between phases.
Detoxification pathways must be balanced to ensure more complete detoxification and elimination and antioxidant support during the phases that generate oxidative stress.
Research suggests that whole foods are preferred to high doses of individual food components, which may have unwanted effects on detoxification enzymes. Whole foods have a balance of compounds that helps modulate detoxification pathways without excessively activating or inhibiting pathways (Hodges 2015):
*Grapefruit juice may interfere with the breakdown of pharmaceuticals and cause a significant drug-nutrient interaction.
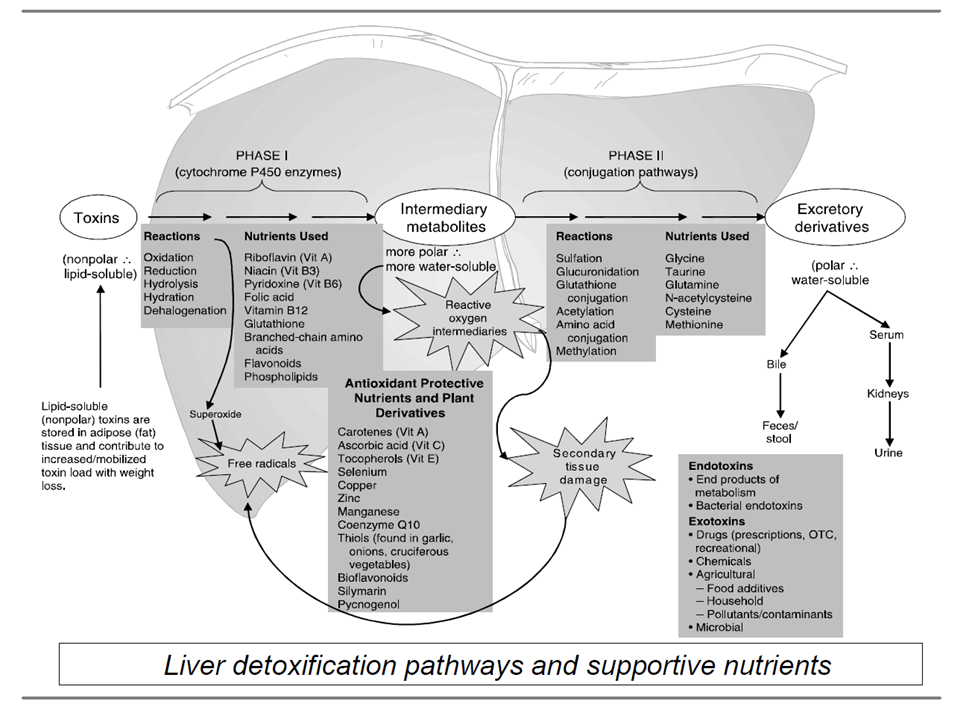
Source: Institute for Functional Medicine. Applying Functional Medicine in Clinical Practice.
Variations in the genes that code for detoxification enzymes, especially the CYP and the GST enzymes, can affect how efficiently someone can break down and eliminate toxins (Raymond 2021).
Single nucleotide polymorphisms (SNPs) that affect phase 1 and 2 detoxification can be identified through advanced testing. However, it has yet to be confirmed that specific variants will have specific detoxification outcomes in humans, especially considering individual factors, including gender, smoking, and lifestyle habits.
Until more definitive guidelines are available, researchers recommend combining functional biomarkers of detoxification, including GGT, homocysteine, and oxidized LDL, with genetic testing and lifestyle assessment when evaluating detoxification competency (Aronica 2022).
Consuming an alkalizing diet can support detoxification, especially the elimination of toxins through the kidney. The most alkalizing foods in general are fruits and vegetables, while the most acid-forming foods are meat, fish, grains, and cheese. Urine pH reflects dietary acid load and can change within two hours of dietary intervention. An alkalizing diet increases urine alkalinity and pH; an increase of 2 points on the urine pH scale can significantly increase the clearance of detoxified compounds. An acidic diet also usurps alkaline reserves throughout the body and compromises cell and tissue metabolism. For example, if the diet is too acidic, the body can counteract that acidity by releasing alkalizing calcium and magnesium from the bone. This process improves acid-base balance at the expense of bone integrity (Minich 2007).
Foods and nutrients that modulate the activity of Phase 1/Phase 2 enzymes and their interaction with genotype.
| Food/Nutrient | Gene | Effects on Enzymatic Function |
|---|---|---|
| Caffeine | CYP1A2 | Caffeine is an inducer and substrate of CYP1A2. rs762551-C allele carriers are “slow” caffeine metabolizers, and they should limit coffee consumption to <1 cup/day or caffeine from other drinks to <100 mg/day to avoid being at higher risks of hypertension and myocardial infarction. In contrast, those with the AA genotype are “rapid” caffeine metabolizers and may benefit from consuming 1–4 cups of coffee/day due to increased consumption of phytonutrients presumed to be protective against heart disease. |
| Cruciferous vegetables (broccoli, Brussels sprouts, cauliflower, watercress, and cabbage) | CYP1A2 | May increase CYP1A2 activity, but it is unclear whether the magnitude of this effect may depend on CYP1A genotype. |
| GSTM1, GSTT1 | Individuals carrying gene deletions in GSTM1 or GSTT1, especially those carrying deletions in both genes, may have a more rapid excretion of bioactive nutrients found in cruciferous vegetables such as isothiocyanates and sulforaphane. Consequently, they may need to consume greater amounts of cruciferous vegetables than those who carry at least one copy of either GSTM1 or GSTT1. On the other side, double-deletion carriers tend to experience a greater increase in GST activity and GST-mediated detoxification upon consumption of cruciferous vegetables or cruciferous-based supplements such as 2-phenethyl isothiocyanate (PEITC). The GST-inducing effects of cruciferous vegetables are more pronounced in females than in males. | |
| UGT1A1 | May decrease serum bilirubin levels in rs3064744-TA allele carriers with greater effects observed for TA/TA homozygous. | |
| Apiaceous vegetables (carrots, celery, dill, parsley, parsnips, etc.) | CYP1A2 | May decrease CYP1A2 activity, but it is unclear whether the magnitude of this effect may depend on CYP1A genotype. May exert inhibitory effects on GSTM-1 in men, not women, who carry at least one copy of the GSTM1 gene. |
| GSTM1, GSTT1 | May exert inhibitory effects on GSTM1 in men, not women, who carry at least one copy of the GSTM1 gene. | |
| UGTA1 | May decrease serum bilirubin levels in rs3064744-TA allele carriers with greater effects observed for TA/TA homozygous. | |
| Quercetin and antioxidant-rich foods (citrus fruits, apples, onions, red wine, olive oil, dark berries, etc.) | CYP1B1 | Quercetin may reduce oxidative stress to a greater extent in rs1056836-G allele carriers than in those with the CC genotype. These findings were made with quercetin from fruit juices at doses significantly lower (~100 mg) than those typically used for supplementation (500–1000 mg). |
| GSTM1, GSTT1 | Quercetin and other antioxidants from blueberry, apples, and purple grapes may reduce oxidative stress to a greater extent in GST double deletion carriers than GST-positive individuals. Smokers who carry GST deletions may especially benefit from supplementation with antioxidants because carcinogens in cigarette smoke can overload their detox capacity and induce a higher production of ROS byproducts. However, quercetin and other antioxidants seem to improve certain oxidative stress markers such as glutathione levels and vitamin C to a greater extent in those with at least one copy of GSTM-1 or GSTT-1. | |
| Tea catechins | COMT | Individuals with the rs4680 AA genotype, who have slow COMT activity, may be slow catechin metabolizers and retain more catechins in the blood than those with the GG genotype. As a result, they may benefit from a lower intake of tea catechins. In contrast, those with the GG genotype, who have higher COMT activity, may be more sensitive to the short-term effects of tea catechins, such as an increase in insulin secretion and blood pressure (BP). |
| Olive oil, red wine | COMT | Individuals with the rs4680 GG genotype, who have higher COMT activity, may experience the health benefits of olive oil and red wine at lower intakes than those with the AA genotype. This is due to a greater ability to convert hydroxytyrosol, a phenolic compound in virgin olive oil and red wine, into its cardioprotective metabolite homovanillyl alcohol (HVAL). |
| Citrus fruit | UGT1A1 | May help lower serum bilirubin in women with the rs3064744 TA/TA genotype. These effects may be noticeable in all TA allele carriers. |
Source: Aronica, Lucia et al. “Genetic Biomarkers of Metabolic Detoxification for Personalized Lifestyle Medicine.” Nutrients vol. 14,4 768. 11 Feb. 2022, doi:10.3390/nu14040768 This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Aronica, Lucia et al. “Genetic Biomarkers of Metabolic Detoxification for Personalized Lifestyle Medicine.” Nutrients vol. 14,4 768. 11 Feb. 2022, doi:10.3390/nu14040768 This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Cline, John C. “Nutritional aspects of detoxification in clinical practice.” Alternative therapies in health and medicine vol. 21,3 (2015): 54-62.
Hodges, Romilly E, and Deanna M Minich. “Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application.” Journal of nutrition and metabolism vol. 2015 (2015): 760689. doi:10.1155/2015/760689
Minich, Deanna M, and Jeffrey S Bland. “Acid-alkaline balance: role in chronic disease and detoxification.” Alternative therapies in health and medicine vol. 13,4 (2007): 62-5.
Noland, Diana, Jeanne A. Drisko, and Leigh Wagner, eds. Integrative and functional medical nutrition therapy: principles and practices. Springer Nature, 2020.
Raymond, Janice L., et al. Krause and Mahan's Food & the Nutrition Care Process. Elsevier, 2021.