This is part 1 of a series of articles we are posting on Insulin Resistance covering the biochemistry and physiology of insulin resistance (IR), IR and the road to diabetes, assessing IR, and steps to reverse IR.
The Biochemistry and Physiology of Insulin
Dicken Weatherby, N.D. and Beth Ellen DiLuglio, MS, RDN, LDN
The ODX Insulin Resistance Series
- Insulin Resistance part 1 - The Biochemistry and Physiology of Insulin
- Insulin Resistance part 2 - Insulin Resistance and the Road to Diabetes
- Insulin Resistance part 3 - Assessing Insulin Resistance - Signs, Symptoms & Individual Biomarkers
- Insulin Resistance part 4 - Take a U-Turn and Reverse Insulin Resistance
- Optimal - The Podcast: Episode 3 Insulin Resistance
Let’s have a brief look at the biochemistry and physiology of insulin.
Insulin is a peptide hormone produced and secreted by the beta cells of the Islets of Langerhans in the pancreas. It has anabolic actions that lower blood glucose and affect lipid and protein metabolism. Insulin promotes synthesis of glycogen, protein, and lipids, and also promotes cell division and growth. [1]
Insulin has a direct effect on [2]
- Muscle
- Increases glucose transport and utilization and stimulates glycogen synthesis and storage
- Liver cells
- Stimulates glycogen synthesis and lipid storage
- Reduces gluconeogenesis and hepatic glucose production
- Decreases availability of circulating fatty acids
- White adipose tissue
- Increases glucose transport and lipogenesis and decreases lipolysis
Pancreatic beta cells are able to store a small amount of insulin that can be released quickly when blood glucose rises.
A reduction in insulin release at this point indicates beta cell dysfunction and predicts an increased likelihood of developing type 2 diabetes.[3] [4]
Insulin resistance
Insulin resistance is considered an early sign of type 2 diabetes development and is characterized by an inability to reduce elevated blood glucose in the face of normal plasma levels of insulin, a phenomenon that often accompanies chronic excess intake of calories, carbohydrates, and fats.
In the event of insulin resistance, target tissues (e.g. muscle, liver, adipose tissue) don’t respond normally to insulin.[5]
Physiologically, there will be a decrease in glucose uptake, glucose oxidation, and glycogen synthesis. There will also be less suppression of lipid oxidation/lipolysis.[6] Endogenous production of glucose will continue and won’t be suppressed as would ordinarily occur with normal insulin sensitivity.
As insulin resistance persists, plasma insulin levels continue to rise to compensate for cellular resistance, eventually leading to lipid and glucose toxicity and beta cell failure.
Research suggests that early signs of subclinical inflammation may precede and predict insulin resistance. This appears to be especially true of hepatic insulin resistance which is associated with early beta cell upregulation. [7] Ultimately, peripheral insulin resistance is the major factor contributing to metabolic syndrome and its progression to type 2 diabetes.[8]
Insulin resistance in general has longed been recognized as a metabolic disorder and risk factor for cardiovascular disease. A detailed review from 1991 notes that [9]
- Insulin resistance is directly related to severity of hypertension and appears to be the connection between essential hypertension and diabetes.
- Even in normal weight individuals, an exaggerated insulin response to glucose is observed in those with hypertension.
- Insulin is noted to be atherogenic as it increases cholesterol transport into arteriole cells where it also enhances lipid synthesis, stimulates cell proliferation, and promotes plaque formation.
At present, insulin resistance is gaining greater recognition as a comorbidity in cardiovascular disease, especially when considering:[10]
- Chronic hyperglycemia contributes to damaging oxidative stress and inflammation.
- Insulin resistance promotes atherogenic dyslipidemia characterized by hypertriglyceridemia, low HDL, and increased small, dense LDL.
- Insulin resistance can directly damage myocardial cells and cause endothelial dysfunction
- Insulin inhibits the use of fatty acids, the heart’s preferred source of energy, which ordinarily provide 50-70% of its ATP needs
A Simplified Model Of Insulin Resistance
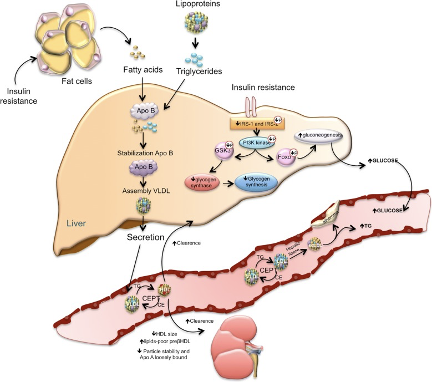
"The loss of suppressive effects of insulin on lipolysis in adipocytes increases free fatty acids. Increased free fatty acids flux to the liver stimulates the assembly and secretion of VLDL resulting in hypertriglyceridemia. Triglycerides (TG) in VLDL are transferred to both HDL and LDL through the action of cholesteryl ester transfer protein (CETP). This process results in a triglyceride-enriched HDL and LDL particle. Triglyceride-enriched HDL is more rapidly cleared from the circulation by the kidney, leaving fewer HDL particles to accept cholesterol from the vasculature. In the glucose metabolism, the insulin resistance results in decreased hepatic glycogen synthesis, owing to decreased activation of glycogen synthase, increased hepatic gluconeogenesis, and glucose delivery by the liver"
Ormazabal, Valeska et al. “Association between insulin resistance and the development of cardiovascular disease.” Cardiovascular diabetology vol. 17,1 122. 31 Aug. 2018.
Insulin resistance is also considered a major risk factor for the development of dyslipidemia, hypertension, and atherosclerosis, and a likely risk factor for stroke and coronary heart disease.[11]
Remember, insulin resistance may be the first sign on the road to type 2 diabetes and other undesirable destinations.
Don’t be afraid to ask for directions, make a U-turn, and get back on the road to optimal health. 
Up Next - Insulin Resistance Part 2: IR and the Road to Diabetes
References
[1] Wilcox, Gisela. “Insulin and insulin resistance.” The Clinical biochemist. Reviews vol. 26,2 (2005): 19-39.
[2] Petersen, Max C, and Gerald I Shulman. “Mechanisms of Insulin Action and Insulin Resistance.” Physiological reviews vol. 98,4 (2018): 2133-2223.
[3] Wilcox, Gisela. “Insulin and insulin resistance.” The Clinical biochemist. Reviews vol. 26,2 (2005): 19-39.
[4] Matthews, D R et al. “Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man.” Diabetologia vol. 28,7 (1985): 412-9.
[5] Petersen, Max C, and Gerald I Shulman. “Mechanisms of Insulin Action and Insulin Resistance.” Physiological reviews vol. 98,4 (2018): 2133-2223.
[6] Ormazabal, Valeska et al. “Association between insulin resistance and the development of cardiovascular disease.” Cardiovascular diabetology vol. 17,1 122. 31 Aug. 2018.
[7] Herder, Christian et al. “Biomarkers of subclinical inflammation and increases in glycaemia, insulin resistance and beta-cell function in non-diabetic individuals: the Whitehall II study.” European journal of endocrinology vol. 175,5 (2016): 367-77.
[8] Adeva, María M et al. “Insulin resistance and the metabolism of branched-chain amino acids in humans.” Amino acids vol. 43,1 (2012): 171-81.
[9] DeFronzo, R A, and E Ferrannini. “Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease.” Diabetes care vol. 14,3 (1991): 173-94.
[10] Ormazabal, Valeska et al. “Association between insulin resistance and the development of cardiovascular disease.” Cardiovascular diabetology vol. 17,1 122. 31 Aug. 2018.
[11] Gutch, Manish et al. “Assessment of insulin sensitivity/resistance.” Indian journal of endocrinology and metabolism vol. 19,1 (2015): 160-4.






