Mold is a type of fungus suspected of causing illness in humans, including asthma, allergic fungal sinusitis, bronchopulmonary aspergillosis (affecting those with asthma and cystic fibrosis), and hypersensitivity pneumonitis (HP), which is less common. Adverse reactions to mold exposure may be due to direct infection with the microorganism, allergic/hypersensitivity immune response generated by the host, and/or exposure to toxic mold byproducts (Bush 2006).
Mold may not be visible until sporulation at which time colorful spores can be detected, e.g., blue/green (Penicilliums), red (Aspergillus, Fusarium, and Rhodotorula), grey (Botrytis), and black (Stachybotrys). Mycotoxins produced by mold and absorbed through the nose, lungs, and skin, can also cause adverse reactions. An estimated 15% of the workforce, as well as 15% of school children may be affected by mold exposure. However, symptoms and adverse effects depend on the intensity of exposure, individual medical history, physiological and psychological stress factors, and nutritional status, especially vitamin D insufficiency (Theoharides 2018).
Phenomena such as chronic inflammatory response syndrome (CIRS) and dampness and mold hypersensitivity syndrome (DMHS) have been associated with mold exposure. They are characterized by innate and adaptive immune responses, a pro-inflammatory state, and multiorgan symptoms (Daschner 2017).
Mold exposure is suspected of contributing to sick-building syndrome (SBS) and related health issues, including skin, mucosal, and general symptomatology. These symptoms, in turn, are associated with clinical biomarkers of inflammation and allergy, including elevated C-reactive protein (CRP), eosinophil counts, and immunoglobulin E (IgE) (Sahlberg 2012).
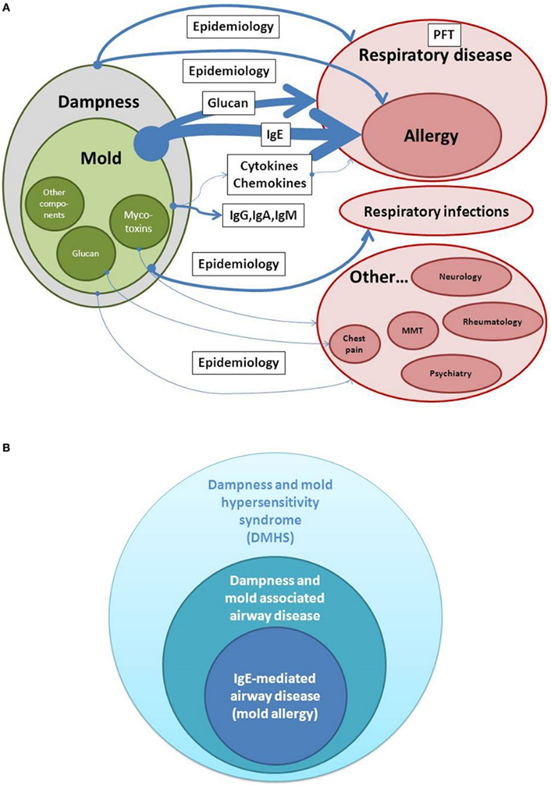
Estimates of evidence for a causal relationship between dampness/mold exposure and disease. Whereas detection of IgE is used as a biomarker in allergic airway disease and belongs to the highest evidence in dampness and mold hypersensitivity syndrome (DMHS), extra-respiratory symptoms are less consistent when analyzed in different epidemiologic studies. PFT, Pulmonary function testing; MMT, Mixed mold mycotoxicosis.
Source: Daschner, Alvaro. “An Evolutionary-Based Framework for Analyzing Mold and Dampness-Associated Symptoms in DMHS.” Frontiers in immunology vol. 7 672. 9 Jan. 2017, doi:10.3389/fimmu.2016.00672. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).
Mold is a significant source of indoor and outdoor air pollution. Climate change, increased humidity, and high carbon dioxide concentrations can increase mold spore production and allergenicity, especially for the common mold Alternaria alternata (Haahtela 2013). An increase in global temperatures of just 2 degrees Celsius favors not only mold growth but also production of harmful aflatoxins (Theoharides 2018). Additional factors that enhance mold growth and mycotoxin exposure include poor management of agricultural commodities and natural disasters (Mehrzad 2021).
The most encountered indoor molds include Cladosporium, Penicillium, and Aspergillus species. Mold thrives where there is a lot of moisture and can grow on a wide variety of surfaces including wood, paper, paint, wallpaper, drywall, carpet, and fabric. Mold can be found in abundance in dust as well (CDC 2022). Some molds, e.g., Aspergillus species, are ubiquitous in both indoor and outdoor settings, and development of infection likely depends on the immune competence of the exposed individual (Bush 2006).
Conditions favorable to fungal growth can increase mold concentrations, especially with poorly maintained structures and improperly repaired leaks. Indoor mold exposure is associated with coughing, wheezing, and rhinitis symptoms in adults and children. Exposure to moisture damage in schools has been associated with nasal symptoms, coughing, wheezing, and absence from school in children. Specific exposure to Alternaria alternata, a mold found indoors and outside, is associated with increased symptoms and prevalence of asthma. Mycotoxins and inflammatory fungal compounds, including beta-1,3-D-glucan (BDG), have also been associated with coughing, nasopharyngeal irritation, and eye irritation (Burbank 2023). Of ten fungal species tested in a study of indoor dust samples, the mold Cladosporium herbarum had the highest BDG content which correlated with prevalence of the microbe. Researchers suggest that concentration of BDG may be a surrogate measure of the prevalence of mold in a specific environment (Iossifova 2007).
Large doses of mycotoxins can occur from ingesting food that has been contaminated or spoiled. However, low doses may not cause illness and not all mold species produce toxins. Aflatoxin and ocratoxin are common mycotoxins found in grains, wine, and peanuts, though regulations are in place to surveil and limit the amount allowed in foods (Bush 2006). Aflatoxin and ocratoxin are considered human carcinogens and chronic consumption is associated with liver and kidney tumors (Redd 2002). Aflatoxin crosses the placenta and makes its way into breast milk and nursing infants (Githang'a 2019).
Mold exposure is associated with varying degrees of illness including invasive mycoses and mold-associated hypersensitivity syndromes. The most frequent cause of mold-related infections are Aspergillus species while the most common non-Aspergillus offenders belong to the Mucorales order which are associated with hypersensitivity pneumonitis (Page 2020).
Mold exposure has long been suspected of causing allergic rhinitis and can exacerbate breathing difficulties in chronic respiratory diseases, including COPD and asthma (Redd 2002).
Exposure to mold in the workplace has been associated with sick-building syndrome (SBS), a phenomenon marked by headache, fatigue, and irritation of the nose, throat, eyes, hands, facial skin, or upper respiratory tract, symptoms that improve when away from the workplace. Data from the 10-year prospective European Community Respiratory Health Survey (ERHC) found that a higher cumulative exposure to building dampness, water leakage, and visible indoor mold growth was associated with decreased remission of symptoms. Symptoms evaluated via questionnaire included dermal, mucosal, and general symptoms, with the latter two symptom types being most prevalent (Zhang 2012):
Further evaluation of data from the ERHC notes that general and mucosal symptoms were higher in those with pre-existing asthma and in women. The incidence of general symptoms also increased with indoor painting (Sahlberg 2012). Researchers note that floor dampness observed in SBS is also associated with toxins released from water-based glue and phthalates from plastic floor covering (Zhang 2012).
|
Genus/Class |
Health Effects |
|
Alternaria
|
Allergen and asthmagen Opportunistic pathogen, opportunistic infections primarily of skin, eye and nose |
|
Alternaria toxin |
Limited data; parenteral data suggest may have development |
|
Aspergillus |
Opportunistic invasive infections Superficial infection of nose, skin, ears, nails; sinusitis; aspergillosis. |
|
Aspergillus toxin Aflatoxin (1) |
(1) Liver, immune, cancer; limited data suggest skin and eye irritant Various other toxins |
|
Chaetomium |
Opportunistic pathogen Infection of skin, ails, and cornea –usually opportunistic |
|
Chaetomium toxin Chetoglobosins |
Limited data: Possible liver, immune, and developmental effects, but data inadequate. Many minor toxins |
|
Cladosporium |
Opportunistic pathogen Infection of skin, nails, and cornea. No toxins |
|
Dicyma |
Maxillary Sinusitis Minor toxins, no data |
|
Epicoccum |
Infection of skin and lung. Suggested allergen and asthmagen |
|
Malassezia |
Common on skin (healthy and diseased) Opportunistic Pityriasis versicolor– hyperpigmentation; psoriasis, seborrheic dermatitis, dandruff, eczema, nail infection. Allergen and asthmagen No toxins |
|
Penicillium |
Opportunistic Superficial infection of nose, skin, ears, nails Allergen and asthmagen |
|
Penicillium toxin
|
Citrinin: Kidney, liver; nasal irritant (limited data), eye irritant Ochratoxin: Kidney, liver, immune, neurological, reproductive, developmental, cancer |
|
Phoma |
Opportunistic Allergen and asthmagen Various minor toxins- No data |
|
Stachybotrys |
No infections, all effects due to toxins; |
|
Stachybotrys toxin Cyclosporine (1)
|
(1) Immune, kidney, liver, cancer Various other toxins |
|
Rhizopus |
Opportunistic, Infections of nose, No toxins |
Exposure to mold, including exposure to dampness suspected of being mold-related, has been associated with increased serum immune and inflammatory markers.
Data from the 10-year ERHC study found that dampness, water leakage, and mold odor in the workplace were associated with increased CRP and total IgE, as well as elevations in eosinophil count, and eosinophil cationic protein (ECP), indicators of eosinophilic inflammation. Prior research revealed an association of SBS with elevated serum myeloperoxidase (MPO), a marker for neutrophilic inflammation (Zhang 2012).
The presence of IgE in serum is an indicator of an allergic immune response. An estimated 10% of the population will generate IgE antibodies to common inhalant molds, though only half of these individuals are expected to have an allergic reaction upon exposure (Bush 2006). Elevated IgE antibodies to mold is a common finding in patients with allergic rhinitis and atopic dermatitis, and allergic reaction to inhaled mold is widely considered a factor in asthma and other lower airway disease. Research indicates that children exposed to indoor mold or dampness were more likely to experience coughing and wheezing than children who were not. Researchers note that while IgE measurement is valuable in assessing mold-related disorders, IgG measurement may only be of value in specific cases, e.g., suspected hypersensitivity pneumonitis or allergic bronchopulmonary mycosis coupled with clinical and historical evidence of exposure. Measurement of IgA and IgM antibodies to molds or antibodies to mycotoxins may not be clinically useful (Bush 2006).
Analysis of biomarkers and respiratory symptoms was carried out in 46 individuals with known mold exposure and 23 without exposure. Results demonstrated significantly higher total IgE in those exposed and significantly higher specific IgE to mold sample mx1, containing Aspergillus fumigatus, Penicillium chrysogenum, Cladosporium herbarum, and Alternaria alternata. Results confirmed earlier findings that IgE testing with mx1 mold mixture is a valid screening tool for assessing potential mold sensitization. IgG testing was not found to significantly identify sensitization to mold. Earlier research also found that elevated sIgE to Aspergillus fumigatus was associated with a 2.7 greater risk of asthma. Previous research also suggests that additional biomarkers may be useful, including IL-6, serum amyloid A (SAA), and CRP, which may be elevated with mold exposure, and significantly decreased levels of anti-inflammatory club cell protein 16 (CC16). Decreased CC16 is also seen in asthmatics and tobacco smokers. The current study found that asthmatics were significantly more likely to be sensitized to molds than non-asthmatics, i.e., 55% versus 18% (Kespohl 2022). It should be noted that some individuals may be hypersensitive to the presence of mold while others may be asymptomatic, and that some mold species, such as Aspergillus, may be able to colonize humans without causing infection (Daschner 2017).
Exposure to mold may not always translate into sensitization or related pathologies, e.g., allergic fungal rhinosinusitis, asthma, and hypersensitivity pneumonia. Although serum antibodies to mold may be valuable for detecting exposure, they cannot determine dose, time, or length of exposure. Researchers suggest that evaluating T-cell frequencies can be useful in the clinical assessment of mold exposure. Research indicates that the number of A. fumigatus-specific T-helper cells was elevated in subjects with intensive mold exposure. Increased mold-specific T-cell counts have been observed in known mold infections such as invasive pulmonary aspergillosis or mucormycosis. However, they may also be detected in healthy subjects in correlation with work or residential mold exposure. One study found that frequencies of T-helper cells specific to A. fumigatus and Mucorales were significantly greater in those who reported high versus low mold exposure. Measuring cytokine response patterns may also assist in the evaluation of mold exposure, including elevations in IFN-gamma, TNF-alpha, IL-5, IL-13, and IL-17. However, researchers suggest further clinical studies to validate these tools (Page 2020).
Optimal protection from mold infection may depend on a balance Th1 and Th2 immune responses. A Th2 immune response often results in an allergic response or susceptibility to infection, while a Th1 immune response is required to clear a fungal infection. The principal T helper subsets contributing protective immunity from pathogenic fungi are Th1 and Th17 (Daschner 2017).
Albumin is able to bind mycotoxins and create adducts that are measurable in serum. For example, the potent S. chartarum toxin satratoxin G (SG) binds prolifically with albumin to create SG-albumin adducts that can be measured in serum (Daschner 2017). Serum albumin adducts are currently measured to gauge exposure to aflatoxin B1 as well as other toxic xenobiotics and chemicals (Yike 2006). Data from the 1999-2000 NHANES study found detectable levels of aflatoxin B1-lysine adduct in just 1% of the US population, despite an expected spike due to weather and agricultural factors (Schleicher 2013). Concentrations of mycotoxins and their metabolites in blood and urine may reflect total body burden and can be useful in assessing mold exposure (Franco 2021).
The contents of nasal lavage fluid can also be evaluated when assessing mold exposure. One study of 12 subjects exposed to a damp water-damaged building observed elevated levels of albumin and myeloperoxidase (MPO) in nasal lavage specimens. Microorganisms detected in the water-damaged building, especially in the gypsum wallboard, included Stachybotrys and Penicillium mold species, as well as bacteria and yeast species (Walinder 2001). Increased TNF-alpha, IL-6, and nitrate levels in nasal lavage fluid have also been associated with exposure to a moldy building (Zhang 2012).
|
Increased |
Decreased |
|
Albumin-mycotoxin adducts CRP Cytokines: IFN-gamma, TNF-alpha, IL-5, IL-13, and IL-17. Eosinophils IgE, total and specific MPO SAA |
CC16 |
Evaluation of general inflammation markers may also enhance the clinical picture when assessing for mold exposure. These include elevations in the following:
Ai, L., Mu, S., & Hu, Y. (2018). Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer cell international, 18, 61. https://doi.org/10.1186/s12935-018-0558-3
Balta S, Celik T, Mikhailidis DP, et al. The Relation Between Atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clin Appl Thromb Hemost. 2016 Jul;22(5):405-11.
Burbank, Allison J et al. “Environmental justice and allergic disease: A Work Group Report of the AAAAI Environmental Exposure and Respiratory Health Committee and the Diversity, Equity and Inclusion Committee.” The Journal of allergy and clinical immunology vol. 151,3 (2023): 656-670. doi:10.1016/j.jaci.2022.11.025
Bush, Robert K., et al. "Environmental and occupational respiratory disorders Position paper The medical effects of mold exposure." J Allergy Clin Immunol 117 (2006): 326-33.
CDC. Basic Facts About Mold and Dampness. 2022.
Consumer Product Safety Commission. CPSC Staff Statement on Toxicology Excellence for Risk Assessment (TERA) Report “Review of the Health Risks of Mold, Health Effects of Molds and Mycotoxins” 2015.
Empting, L D. “Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure.” Toxicology and industrial health vol. 25,9-10 (2009): 577-81. doi:10.1177/0748233709348393
Farwell, Wildon R, and Eric N Taylor. “Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey.” CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne vol. 182,2 (2010): 137-41. doi:10.1503/cmaj.090329
Githang'a, David et al. “The effects of exposures to mycotoxins on immunity in children: A systematic review.” Current problems in pediatric and adolescent health care vol. 49,5 (2019): 109-116. doi:10.1016/j.cppeds.2019.04.004
Gonzalez-Quintela, A et al. “Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities.” Clinical and experimental immunology vol. 151,1 (2008): 42-50. doi:10.1111/j.1365-2249.2007.03545.x
Haahtela, Tari et al. “The biodiversity hypothesis and allergic disease: world allergy organization position statement.” The World Allergy Organization journal vol. 6,1 3. 31 Jan. 2013, doi:10.1186/1939-4551-6-3
Iossifova, Y., C. Crawford, and T. Reponen. "Content of (1-3)-β-D-glucan in Common Indoor Air Fungi." Journal of Allergy and Clinical Immunology 119.1 (2007): S186.
Kespohl, Sabine et al. “What should be tested in patients with suspected mold exposure? Usefulness of serological markers for the diagnosis.” Allergologie select vol. 6 118-132. 29 Mar. 2022, doi:10.5414/ALX02298E This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Madsbad, Sten. “Impact of postprandial glucose control on diabetes-related complications: How is the evidence evolving?.” Journal of diabetes and its complications vol. 30,2 (2016): 374-85. doi:10.1016/j.jdiacomp.2015.09.019
Malavolta, Marco et al. “Serum copper to zinc ratio: Relationship with aging and health status.” Mechanisms of ageing and development vol. 151 (2015): 93-100. doi:10.1016/j.mad.2015.01.004
Mehrzad, Jalil. "Environmentally relevant level of aflatoxin B1 and the role of (non) oxidative immuno-/neurodysregulation and toxicity." Toxicology. Academic Press, 2021. 165-179.
Nilsson, Bo et al. “C3 and C4 are strongly related to adipose tissue variables and cardiovascular risk factors.” European journal of clinical investigation vol. 44,6 (2014): 587-96. doi:10.1111/eci.12275
Pagana, Kathleen Deska, et al. Mosby's Diagnostic and Laboratory Test Reference. 15th ed., Mosby, 2021.
Page, Lukas, et al. "3.1 Evaluation of Aspergillus and Mucorales specific T-cells and peripheral blood mononuclear cell cytokine signatures as biomarkers of environmental mold exposure." Entwicklung und präklinische Evaluation immunologischer und nuklearmedizinischer diagnostischer Tests für Schimmelpilz-assoziierte Hypersensitivität und invasive Mykosen (2020): 64.
Quest Diagnostics. Allergy Mold Panel, Complete. https://testdirectory.questdiagnostics.com/test/test-detail/92170/allergy-mold-panel-complete?cc=MASTER
Quest Diagnostics. Mold Panel #1. https://testdirectory.questdiagnostics.com/test/test-detail/37922/?cc=AMD
Redd, Stephen C. "State of the science on molds and human health." Center for Disease Control and Prevention, US Department of Health and Human Services 1.11 (2002).
Sahlberg, B et al. “Onset of mucosal, dermal, and general symptoms in relation to biomarkers and exposures in the dwelling: a cohort study from 1992 to 2002.” Indoor air vol. 22,4 (2012): 331-8. doi:10.1111/j.1600-0668.2012.00766.x
Tavora, Fabio R et al. “Monocytes and neutrophils expressing myeloperoxidase occur in fibrous caps and thrombi in unstable coronary plaques.” BMC cardiovascular disorders vol. 9 27. 23 Jun. 2009, doi:10.1186/1471-2261-9-27
Theoharides, Theoharis C. "Mold and immunity." Clinical Therapeutics 40.6 (2018): 882-884.
Wålinder, R et al. “Nasal lavage biomarkers: effects of water damage and microbial growth in an office building.” Archives of environmental health vol. 56,1 (2001): 30-6. doi:10.1080/00039890109604052
Yang, Ming et al. “The gamma gap predicts 4-year all-cause mortality among nonagenarians and centenarians.” Scientific reports vol. 8,1 1046. 18 Jan. 2018, doi:10.1038/s41598-018-19534-4
Yike, Iwona et al. “Mycotoxin adducts on human serum albumin: biomarkers of exposure to Stachybotrys chartarum.” Environmental health perspectives vol. 114,8 (2006): 1221-6. doi:10.1289/ehp.9064
Zahorec, R. “Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill.” Bratislavske lekarske listy vol. 102,1 (2001): 5-14.
Zhang, Xin et al. “Dampness and moulds in workplace buildings: associations with incidence and remission of sick building syndrome (SBS) and biomarkers of inflammation in a 10 year follow-up study.” The Science of the total environment vol. 430 (2012): 75-81. doi:10.1016/j.scitotenv.2012.04.040