Thyroid hormones take a front and center role in regulating metabolism, directly affecting energy expenditure, weight maintenance, lipid metabolism, gluconeogenesis, and growth and development.[1]
The thyroid hormone thyroxine (T4) is technically a prohormone with very little functional activity of its own. The metabolically active form is triiodothyronine (T3) and, as its name implies, it contains three molecules of iodine (triiodo-). It is produced when one iodine molecule is removed from T4.[2] [3]
Although a small amount of T3 is produced within the thyroid gland, most is derived from the conversion of T4 to T3. This conversion occurs primarily in the liver, kidney, and thyroid but also takes place in the brain, pituitary, intestine, skin, brown adipose tissue, bone, endothelial cells, and skeletal muscle. [4][5]
Most circulating thyroid hormones are bound to proteins as they travel through the bloodstream and can be measured as total T4 and total T3.[6] However, only free unbound hormone is available for use. For example, T3 must be released from serum proteins as free T3 before it can exert its effects in target tissues. Measuring both free T3 (FT3) and free T4 (FT4) helps assess actual hormone availability.
Calculating the ratio of FT3 to FT4 can provide further information about current thyroid status. The FT3:FT4 ratio reflects conversion of T4 to T3. This process is dependent on selenium-containing deiodinase enzymes, making selenium a key player in thyroid function right alongside iodine.[7] Adequate selenium intake of 60-75 ug/day is needed to maintain selenoenzyme activity and prevent destructive autoimmune activity in the thyroid gland.[8]
Inflammation can disrupt deiodinase activity, alter thyroid hormone levels, and affect ratios. Under certain circumstances, a specialize deiodinase will convert T4 to reverse T3 to curb metabolism. Such conditions include chronic inflammation, critical illness, stress, myocardial infarction, cardiac hypertrophy, cancer, and starvation.[9]
An elevated FT3:FT4 ratio, reflecting increased free T3 and/or decreased free T4, can be associated with a variety of conditions. These include Graves’ disease, metabolic syndrome, insulin resistance, and fatty liver.[10] [11] [12]
An increased FT3:FT4 can also be an indicator of cardiovascular risk as it positively correlates with pulse wave velocity and adipose-related cardiovascular inflammatory markers interleukin-6 and high-sensitivity CRP.[13]
Research suggests that an elevated FT3:FT4 ratio may be predictive of metabolic syndrome. Results from the Dutch population-based LifeLines Cohort study of 26,719 men and women revealed that increases in the FT3:FT4 ratio were associated with four of five defining components of metabolic syndrome (i.e. blood pressure, HDL-cholesterol, triglycerides, and waist circumference but not fasting blood glucose). Subjects in the highest quartiles of FT3:FT4 ratio had a 50-80% increased risk of metabolic syndrome than those in the lowest quartile.[14] Mean baseline FT3:FT4 ratio was 2.88 for men and 2.75 for women (FT3 in pg/mL and FT4 in ng/dL).
A cross-sectional study of 132,346 male and female euthyroid participants within a single institution determined that both FT3:FT4 ratio and TSH were positively associated with markers for insulin resistance and parameters of metabolic syndrome. Specifically, FT3:FT4 positively correlated with HOMA-IR, waist circumference, triglyceride levels, fasting blood glucose, and systolic blood pressure. Interestingly, the FT3 to FT4 ratio had a stronger association with metabolic syndrome risk than did TSH.[15] Highest values for FT3:FT4 ratio were 2.98 or higher for men and 2.89 or higher for women (FT3 in pg/mL, FT4 in ng/dL) and 0.358 or higher for men, 0.346 or higher for women using SI pmol/L for both FT3 and FT4.
Analysis of the FT3:FT4 ratio in an ongoing population study of male and female euthyroid adults revealed a close association between an increasing ratio, metabolic anomalies, and cardiovascular risk factors.[16]
In this study, FT3:FT4 ratio positively correlated with characteristic components of metabolic syndrome such as waist circumference, waist-to-hip ratio, BMI, blood pressure, triglyceride levels, and fasting blood glucose. It also correlated positively with carotid-femoral pulse wave velocity, IL-6, and hs-CRP. The ratio was negatively correlated with HDL-cholesterol. The mean FT3:FT4 ratio was 2.5 for men and 2.3 for women using FT3 in pg/mL, FT4 in ng/dL. The mean ratios using pmol/L for FT3 and FT4 were 0.3 for men and 0.29 for women.
A reduced FT3:FT4 ratio may indicate the use of T4 only therapy, hypothyroidism, selenium deficiency, disrupted deiodinase activity, and reduced production of T3 and free T3. A ratio of less than 2 suggests the presence of low T3 syndrome.[17]
Critical illness, inflammation, and hypoxia may interfere with the conversion of T4 to T3 and increase degradation of T4, essentially leading to a rise in FT3:FT4 ratios.
Low free T3 can be a sensitive marker of ill health and both a decreased FT3 and an increased FT4 correlate with acute and chronic disease and help predict long-term mortality risk. Results from a retrospective cohort analysis of male and female adults revealed that those with a lower FT3:FT4 ratio of 2.1 (FT3 in pg/mL and FT4 in ng/dL) were at increased risk of mortality during a follow-up period of 36 months. It was noted that CRP, sedimentation rate, ferritin, and LDH were also elevated in the mortality group.[18]
The FT3:FT4 ratio can be useful in assessment of cardiovascular risk as well. The heart and cardiovascular system are profoundly affected by thyroid hormones. Subclinical hypothyroidism is strongly correlated with atherosclerosis, atrial fibrillation, infarct size, and increased mortality in cardiac disease. The relationship between FT3:FT4 ratio and cardiovascular mortality was investigated in a prospective cohort study of 953 euthyroid male and female adult undergoing percutaneous coronary intervention after acute MI.[19] The study observed that a decreasing FT3:FT4 ratio increased likelihood of 1-year all-cause mortality by 255%.
Since both low T3 and selenium insufficiency contribute to heart disease,[20] the association should be studied further in order to enhance the diagnostic value of readily available biomarkers and optimize clinical and nutrition interventions.
So, bottom line, always remember to check your spark plugs first!
Good news! The Free T3: Free T4 ratio is now being automatically calculated by the Optimal DX software if both the Free T3 and Free T4 are added into the system. No need to do any conversions as the software will do this for you and will now show the result in the Blood Test Results Report:
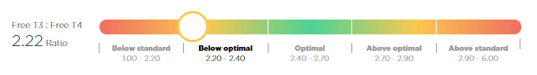
However, if you simply want to do the calculation manually then please follow these instructions:

[1] Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014 Apr;94(2):355-82 [R]
[2] Larsen PR, Zavacki AM. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur Thyroid J. 2012;1(4):232-242. [R]
[3] Karapanou, O., & Papadimitriou, A. (2011). Thyroid hormone transporters in the human. HORMONES, 10(4), 270-279.
[4] Peeters RP, Visser TJ. Metabolism of Thyroid Hormone. 2017 Jan 1. In: Feingold KR, Anawalt B, Boyce A, et al, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from [R]
[5] Larsen PR, Zavacki AM. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur Thyroid J. 2012;1(4):232-242. [R]
[6] Werner, Sidney C., and Ingbar, Sidney H.. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. United Kingdom, Wolters Kluwer Health, 2012.
[7] Köhrle J. Selenium and the control of thyroid hormone metabolism. Thyroid. 2005 Aug;15(8):841-53. [R]
[8] Lacka K, Szeliga A. Significance of selenium in thyroid physiology and pathology. Pol Merkur Lekarski. 2015 Jun;38(228):348-53. [R]
[9] Gomes-Lima C, Wartofsky L, Burman K. Can Reverse T3 Assay Be Employed to Guide T4 vs. T4/T3 Therapy in Hypothyroidism? Front Endocrinol (Lausanne). 2019 Dec 11;10:856. [R]
[10] Sriphrapradang C, Bhasipol A. Differentiating Graves' disease from subacute thyroiditis using ratio of serum free triiodothyronine to free thyroxine. Ann Med Surg (Lond). 2016 Aug 8;10:69-72. [R]
[11] Gökmen FY, Ahbab S, Ataoğlu HE, et al. FT3/FT4 ratio predicts non-alcoholic fatty liver disease independent of metabolic parameters in patients with euthyroidism and hypothyroidism. Clinics (Sao Paulo). 2016 Apr;71(4):221-5. [R]
[12] van den Berg EH, van Tienhoven-Wind LJ, Amini M, et al. Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: the Lifelines Cohort Study. Metabolism. 2017 Feb;67:62-71. [R]
[13] Roef GL, Rietzschel ER, Van Daele CM, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014 Feb;24(2):223-31. [R]
[14] Wolffenbuttel BHR, Wouters HJCM, Slagter SN, et al Thyroid function and metabolic syndrome in the population-based LifeLines cohort study. BMC Endocr Disord. 2017 Oct 16;17(1):65. [R]
[15] Park SY, Park SE, Jung SW, et al. Free triiodothyronine/free thyroxine ratio rather than thyrotropin is more associated with metabolic parameters in healthy euthyroid adult subjects. Clin Endocrinol (Oxf). 2017 Jul;87(1):87-96. [R]
[16] Roef GL, Rietzschel ER, Van Daele CM, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014 Feb;24(2):223-31. [R]
[17] Nomura R, Miyai K, Kuge R, Okura T, Goto M, Hasegawa Y. Free T3 to free T4 ratio less than 2.0 suggests low T3 syndrome rather than central hypothyroidism from the age of two to eighteen years. Endocr J. 2017 Feb 27;64(2):213-219. [R]
[18] Ataoğlu HE, Ahbab S, Serez MK, et al. Prognostic significance of high free T4 and low free T3 levels in non-thyroidal illness syndrome. Eur J Intern Med. 2018 Nov;57:91-95. [R]
[19] Yu T, Tian C, Song J, et al. Value of the fT3/fT4 ratio and its combination with the GRACE risk score in predicting the prognosis in euthyroid patients with acute myocardial infarction undergoing percutaneous coronary intervention: a prospective cohort study. BMC Cardiovasc Disord. 2018 Sep 10;18(1):181. [R]
[20] Schomburg L, Orho-Melander M, Struck J, Bergmann A, Melander O. Selenoprotein-P Deficiency Predicts Cardiovascular Disease and Death. Nutrients. 2019 Aug 9;11(8):1852. [R]